Suggested `plantTracker` Workflow
18 February 2022
Source:vignettes/Suggested_plantTracker_Workflow.Rmd
Suggested_plantTracker_Workflow.RmdIntroduction
Welcome to plantTracker! This package was designed to
transform long-term quadrat maps that show plant occurrence and size
into demographic data that can be used to answer questions about
population and community ecology. This vignette is designed to help you
use plantTracker functions to move from a spatially
referenced dataset containing plant cover data, to an output dataset
that contains growth and survival data for each observed individual.
This vignette will walk you through the steps to get from maps of plant cover to a demographic dataset that you can use for analysis!
1. Prepare data
The functions in plantTracker require data in a specific
format. plantTracker includes an example dataset that
consists of two pieces: grasslandData and
grasslandInventory. You can load these example datasets
into your global environment by calling data(grasslandData)
and data(grasslandInventory). You can view the
documentation for these datasets by calling ?grasslandData
and ?grasslandInventory.
Most plantTracker functions require two data objects.
The first is a data frame that contains the location and metadata for
each mapped individual, which we from now on will call dat.
The second is a list that contains a vector of years in which each
quadrat was sampled, which we from now on will cal inv.
Below are the basic requirements for these data objects.
1.1 The dat data frame must . . .
- … be an
sfdata.frame. More on this below in section 1.1.1… - … contain a row for each individual observation in each year.
- … have a column that contains character strings indicating the specific epithet for each observation. This column must be a character data type. The function expects this column to be called “Species”, but a different name can be specified in function calls.
- … have a column that contains character strings indicating the site at which each observation was collected. This is a level of classification “above” the quadrat (i.e. all quadrats measured at the Central Plains Experimental Range in Nunn, CO might have the value “CO” in this column). This column must be a character data type. The function expects this column to be called “Site”, but a different name can be specified in function calls.
- … have a column that contains character strings indicating the quadrat at which each observation was collected. This column must be a character data type. The function expects this column to be called “Quad”, but a different name can be specified in function calls.
- … have a column that contains a value indicating the year when this individual observation was collected. This must be a numeric data type, and must be either a four or two digit year number. The function expects this column to be called “Year”, but a different name can be specified in function calls.
- … have a column (almost always called “geometry” in the
sfpackage data format) that contains a polygon representing the location of each observation. Each observation must be aPOLYGONorMULTIPOLYGON. Data cannot be stored asPOINTS.- If the data was collected such that forbs or small grasses were mapped as points and digitized as such, then these observations must be converted to polygons. We recommend that you convert them to small circular polygons with an identical radius. If you do this transformation, we also recommend that you include a column that indicates whether each row was originally mapped as a polygon or a point, since the demographic data that deals with size will be relatively meaningless for observations originally mapped as points.
-
datdoes not need to have a coordinate reference system (i.e. CRS can be “NA”), but it can have one if you’d like.
- … not have columns called “neighbors”, “nearEdge”,
“trackID”, “age”, “size_tplus1”, “recruit”, “survives_tplus1”,
“basalArea_ramet”, or “basalArea_genet”, since these columns are added
by
plantTrackerfunctions. - Note: Additional columns can be included in the input data.frame,
although they might not be included in the output of
plantTrackerfunctions.
Here are the first few rows of a possible dat input
data.frame:
#> Simple feature collection with 6 features and 6 fields
#> Geometry type: POLYGON
#> Dimension: XY
#> Bounding box: xmin: -0.000160084 ymin: 0.4334812 xmax: 0.286985 ymax: 0.9419673
#> CRS: NA
#> Species Type Site Quad Year sp_code_6
#> 1 Heteropogon contortus poly AZ SG2 1922 HETCON
#> 2 Heteropogon contortus poly AZ SG2 1922 HETCON
#> 3 Heteropogon contortus poly AZ SG2 1922 HETCON
#> 4 Heteropogon contortus poly AZ SG2 1922 HETCON
#> 5 Heteropogon contortus poly AZ SG2 1922 HETCON
#> 6 Heteropogon contortus poly AZ SG2 1922 HETCON
#> geometry
#> 1 POLYGON ((0.237747 0.908835...
#> 2 POLYGON ((0.2833037 0.85959...
#> 3 POLYGON ((0.008583123 0.449...
#> 4 POLYGON ((0.1480142 0.46983...
#> 5 POLYGON ((0.03573306 0.5259...
#> 6 POLYGON ((0.2441894 0.52689...- Note: that the required columns are “Species”, “Site”, “Quad”,
“Year”, and “geometry”. The additional columns “Type” and “sp_code_6”
are just “along for the ride” in any analysis using
plantTrackerfunctions.
Here’s what some of the example dat data (from the “SG2”
quadrat at the “AZ” site in 1922) look like when plotted spatially:
#> Warning: Using `size` aesthetic for lines was deprecated in ggplot2 3.4.0.
#> ℹ Please use `linewidth` instead.
#> This warning is displayed once every 8 hours.
#> Call `lifecycle::last_lifecycle_warnings()` to see where this warning was
#> generated.Figure 1.1 : Spatial map of a subset of example ‘dat’ dataset
It’s important to note that, while plantTracker was
designed to be used with small-scale maps of plant occurrence in
quadrats, it is possible to use other styles of map data in
plantTracker functions. All that is required is a single
mapped basal area (or point location converted to a small polygon) at
each time point for each organism (or ramet), and is accompanied by the
required metadata detailed above. For example, plantTracker
functions could be used to estimate tree demographic rates at the scale
of 100 m x 50 m plots.
1.1.1 Get your data into the sf data
format
As mentioned above, plantTracker uses the
sf R package to deal with spatial data. The map data that
plantTracker was built to analyze is inherently spatial, so
you need to know how to the basics of dealing with spatial data in R if
you want to use plantTracker! There are many good resources
to help you orient yourself to working with spatial data in R
generally:
-
https://cengel.github.io/R-spatial/intro.html
- https://www.r-bloggers.com/2021/06/using-geospatial-data-in-r/
And the sf package more specifically:
These resources provide a great orientation, and while I recommend
looking over them if you’re new to working with spatial data in R, I’ve
included a brief tutorial for uploading shapefiles into R as
sf data frames.
Most of the published chart-quadrat datasets have the map data stored
as shapefiles in complex file structures, which can be a bit confusing
to navigate. plantTracker requires all of your data (for
all species, plots and years) to be in one single data frame. This
example shows how you might navigate through a complex file structure to
to pull out shapefiles and put them into one single sf data
frame. for further analysis with plantTracker. For
this example, I’ll use a subset of the data from the Santa Rita
Experimental Range in Arizona, which has been published in this
data paper. In this dataset, shapefiles for each quadrat are stored
in their own folder. Within that folder there are two shapefiles for
each year: one that contains map data for polygons, and one that
contains data for points. The following code reads in those shapefiles,
transforms the points to polygons of a fixed radius, and puts all the
data into one sf data frame. If you want to follow along,
download the “shapefiles.zip” file from the data paper, un-zip it, and
name it “AZ_shapefiles”. The dataset that is the result of this example
is the same as part of the “grasslandData” dataset included in
plantTracker.
# save a character vector of the file names in the file that contains the
# shapefiles (in this case, called "CO_shapefiles"), each of which is a quadrat
# note: 'wdName' is a character string indicating the path of the directory
# containing the 'AZ_shapefiles' folder
quadNames <- list.files(paste0(wdName,"AZ_shapefiles/"))
# trim the quadrats down to 2, for the sake of runtime in this example
quadNames <- quadNames[quadNames %in% c("SG2", "SG4")]
# now we'll loop through the quadrat folders to download the data
for (i in 1:2){#length(quadNames)) {
# get the names of the quadrat for this iteration of the loop
quadNow <- quadNames[i]
# get a character vector of the unique quad/Year combinations of data in
# this folder that contain polygon data
quadYears <- quadYears <- unlist(strsplit(list.files(
paste0(wdName, "AZ_shapefiles/",quadNow,"/"),
pattern = ".shp$"), split = ".shp"))
# loop through each of the years in this quadrat
for (j in 1:length(quadYears)) {
# save the name of this quadYear combo
quadYearNow <- quadYears[j]
# read in the shapefile for this quad/year combo as an sf data frame
# using the 'st_read()' function from the sf package
shapeNow <- sf::st_read(dsn = paste0(wdName,"AZ_shapefiles/",quadNow),
# the 'dsn' argument is the folder that
# contains the shapefile files--in this case,
# the folder for this quadrat
layer = quadYearNow) # the 'layer' argument has the
# name of the shapefile, without the filetype extension! This is because each
# shapefile consists of at least three separate files, each of which has a
# unique filetype extension.
# the shapefiles in this dataset do not have all of the metadata we
# need, and have some we don't need, so we'll remove what we don't need and
# add columns for 'site', 'quad', and 'year'
shapeNow$Site <- "AZs"
shapeNow$Quad <- quadNow
# get the Year for the shapefile name--in this case it is the last for
# numbers of the name
shapeNow$Year <- as.numeric(strsplit(quadYearNow, split = "_")[[1]][2]) + 1900
# determine if the 'current' quad/year contains point data or polygon data
if (grepl(quadYearNow, pattern = "C")) { # if quadYearNow has point data
# remove the columns we don't need
shapeNow <- shapeNow[,!(names(shapeNow)
%in% c("Clone", "Seedling", "Area", "Length", "X", "Y"))]
# reformat the point into a a very small polygon
# (a circle w/ a radius of .003 m)
shapeNow <- sf::st_buffer(x = shapeNow, dist = .003)
# add a column indicating that this observation was originally
# mapped as a point
shapeNow$type <- "point"
} else { # if quadYearNow has polygon data
# remove the columns we don't need
shapeNow <- shapeNow[,!(names(shapeNow) %in% c("Seedling", "Canopy_cov", "X", "Y", "area"))]
# add a column indicating that this observation was originally
# mapped as a polygon
shapeNow$type <- "polygon"
}
# now we'll save this sf data frame
if (i == 1 & j == 1) { # if this is the first year in the first quadrat
dat <- shapeNow
} else { # if this isn't the first year in the first quadrat, simply rbind
# the shapeNow sf data frame onto the previous data
dat <- rbind(dat, shapeNow)
}
}
}
# Now, all of the spatial data are in one sf data frame!
# for the sake of this example, we'll remove data for some species and years in order to make the example run faster (and to make this 'dat' data.frame identical to the "grasslandData" dataset included in this R pakcage).
dat <- dat[dat$Species %in% c("Heteropogon contortus", "Bouteloua rothrockii", "Ambrosia artemisiifolia", "Calliandra eriophylla", "Bouteloua gracilis", "Hesperostipa comata", "Sphaeralcea coccinea", "Allium textile"),]
dat <- dat[ (dat$Quad %in% c("SG2", "SG4") &
dat$Year %in% c(1922:1927)),]In some spatial datasets, observations that were measured as “points”
in the field are still stored as “points” in the shapefiles.
plantTracker requires all observations to be stored as
“polygon” geometry in order to streamline functions, so we need to
translate “points” into small polygons of a fixed area. In this case,
we’ll transform them into circles with a radius of 1 cm (.01, since this
dataset measures area in meters). ‘dat’ has a column called “type.” A
value of “point” in this column will tell us that, even though the
geometry of the “point” data is now in “polygon” format, the values for
basal area and growth are not indicative of the true size of the
plant.
# We use the function "st_buffer()" to add a buffer of our chosen radius (.01) around each point observation, which will transform each observation into a circle of the "polygon" format with a radius of .01.
dat_1 <- st_buffer(x = dat[st_is(x = dat, type = "POINT"),], dist = .01)
dat_2 <- dat[!st_is(x = dat, type = "POINT"),]
dat <- rbind(dat_1, dat_2)If you don’t want to download the data and format it into an sf data.frame, you can also use a subset of the “grasslandData” data object stored in this R package. You will just need to subset it to include only the data from the “AZ” site. Code to do this is below:
dat <- grasslandData[grasslandData$Site == "AZ",]
1.2 The inv list must . . .
- … be a named list
- … have element names that are each a character string identical to
the name of a quadrat in
dat. There cannot be two elements with the same name, and there cannot be an element with more than one quadrat in its name. There must be an element for each quadrat with data indat. - … have list elements that are each a numeric vector of years in
which each quadrat was sampled. If a quadrat was “skipped” in a year,
then that year must be excluded from this vector. The format of the
years must be the same as the format of years in
dat(i.e. if year is a four-digit number indat, then it must be a four-digit number ininv). Make sure this is the years the quadrat was actually sampled, not just the years that have data in thedatdata frame! This argument allows the function to differentiate between years when the quadrat wasn’t sampled and years when there just weren’t any individuals of a species present in that quadrat. If a quadrat wasn’t sampled in a given year, don’t put an ‘NA’ ininvfor that year! Instead, just skip that year.
Here is an example of an inv argument that corresponds
to the example dat argument above. The quadrats that have
data in dat are “SG2” and “SG4”, so there are elements in
inv that correspond to each of these quadrats.
#> $SG2
#> [1] 1922 1923 1924 1925 1926 1927
#>
#> $SG4
#> [1] 1922 1923 1924 1925 1926 1927If you already have a quadrat inventory as a data frame, it isn’t
complicated to reformat it to work with plantTracker
functions. For example, if your quadrat inventory data frame looks like
this… :
#> quad1 quad2 quad3
#> 1 2000 2000 2000
#> 2 2001 2001 NA
#> 3 NA 2002 2002
#> 4 2003 2003 2003
#> 5 2004 2004 2004
#> 6 2005 2005 2005
#> 7 2006 2006 2006
#> 8 2007 2007 2007… then do the following to get it into a format ready for
plantTracker:
quadInv_DF <- data.frame("quad1" = c(2000, 2001, NA, 2003, 2004, 2005, 2006, 2007),
"quad2" = c(2000:2007),
"quad3" = c(2000, NA, 2002, 2003, 2004, 2005, 2006, 2007))
# use the 'as.list()' function to transform your data frame into a named list
quadInv_list <- as.list(quadInv_DF)
# we still need to remove the 'NA' values, which we can do using the
# 'lapply()' function
(quadInv_list <- lapply(X = quadInv_list, FUN = function(x) x[is.na(x) == FALSE]))
#> $quad1
#> [1] 2000 2001 2003 2004 2005 2006 2007
#>
#> $quad2
#> [1] 2000 2001 2002 2003 2004 2005 2006 2007
#>
#> $quad3
#> [1] 2000 2002 2003 2004 2005 2006 2007
1.3 Check the inv and dat
arguments using checkDat()
The generic checkDat() function:
checkDat(dat, inv = NULL, species = "Species", site = "Site", quad = "Quad",
year = "Year", geometry = "geometry", reformatDat = FALSE, ...)This step is optional, but can be useful if you’re unsure whether
your dat and inv arguments are in the correct
format. The plantTracker function checkDat()
takes dat and inv as arguments for the
arguments dat and inv, and will return
informative error messages if either argument is not in the correct
format.
Additional optional arguments to checkDat() are
species, site, quad,
year, geometry, and
reformatDat.
-
species/site/quad/year/geometryThese arguments only need to be included if the columns indatthat contain the data for species, site, quadrat, year and geometry of each observation are different from the names “Species”, “Site”, “Quad”, “Year, and”geometry”. For example, if the column in your version ofdatthat contains the species identity of each observation is called “species_names”, then the argumentspecies = "species_names"must be included in your call tocheckDat(). -
reformatDatis a TRUE/FALSE argument that determines whether you want thecheckDat()function to return a version ofdatthat is ready to go into the steps of this workflow. IfreformatDat= TRUE thencheckDat()will return a list that contains the reformatted version ofdat, the reformatted version ofinvand an additional element called “userColNames”, which contains the column names in the input version ofdatthat are different from the expected column names of “Species”, “Site”, “Quad”, “Year, and”geometry” (if there are any). IfreformatDat= TRUE, thencheckDat()will return a message indicating that your data is ready for the next step. The default value is FALSE.
2 Track individuals through time using
trackSpp()
Now it’s time to transform your raw dataset into demographic data!
This is accomplished using the trackSpp() function. This
function follows individual plants from year to year in the same quadrat
to determine survival, size in the next year, age, and some additional
potentially-useful demographic data. It does this by comparing quadrat
maps from sequential years. If there is overlap of individuals of the
same species in consecutive years, then the rows in dat
that contain data for those overlapping individuals are given the same
“trackID”, or unique identifier.
Here is the generic trackSpp() function:
trackSpp(dat, inv, dorm, buff, buffGenet, clonal, species = "Species",
site = "Site", quad = "Quad", year = "Year", geometry = "geometry",
aggByGenet = TRUE, printMessages = TRUE, flagSuspects = FALSE,
shrink = 0.1, dormSize = 0.05, ...)2.1 Function arguments
trackSpp() takes the following arguments:
datThis is thesfdata frame that we’ve been callingdatso far. This must be in the correct format (which you can check before-hand usingcheckDat()), but informative error messages will be returned if it is incorrect. It must have the columns outlined in Section 1.1, but they can have different names as long as those names are included in this function call (more on that later…).invThis is the list of quadrat sampling years we’ve been callinginv. If it is not in the correct format or does not contain data for the correct quadrats, then an informative error message will be returned.-
dormThis is a positive integer value that indicates how long you want the function to allow an individual to be “dormant”. In this case, dormancy can be interpreted as the biological phenomenon where a plant has above-ground tissue present in year 1, is alive underground but with no above-ground tissue in year 2, and then has above-ground tissue in a subsequent year. Dormancy can also be interpreted here as data-collection error, whereby an individual is accidentally not mapped in between years where it was recorded.Consider the following example: There is a polygon of species “A” in year 1, which is our “focal individual”. In year 2, there is not a polygon of species “A” that overlaps with our focal individual. In year 3, there is a polygon of species “A” that is in the same location as our focal individual. If
dorm = 0, then our focal individual would get a 0 in the survival column, and the polygon of species “A” in year 3 would be considered a new recruit and get a new trackID. Ifdorm = 1, because there is overlap between two polygons of the same species with only a 1-year gap between when they occur, these two polygons will be considered the same genetic individual, will have the same trackID, and our focal individual will have a “1” in the survival column. In an alternative scenario, in years 3 and 4 there are not polygons of species “A” that are in the same location as our focal individual, but there is a polygon in year 4 that overlaps our focal individual. Ifdorm = 1, then our focal individual would get a “0” for survival, but ifdorm = 2, then it would get a 1 for survival.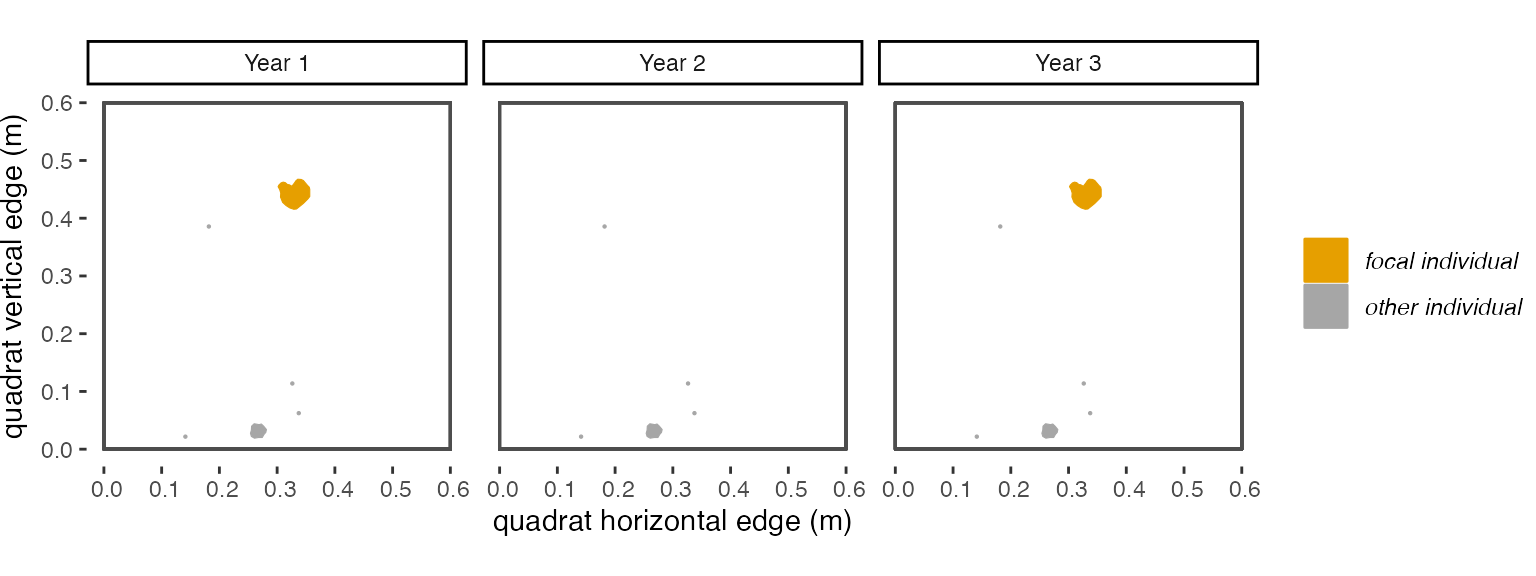
Figure 2.1: A visualization of the ‘dormancy’ scenario described above.
If you’d like to be more specific and perhaps biologically accurate, you can also specify the
dormargument uniquely for each species. For example, it might be that you are confident that your data collectors did not accidentally “miss” any individuals, and yourdatdata frame contains observations for shrubs or trees, which are very unlikely to go dormant, and small forbs, which are much more likely to go dormant for one or two years. In order to disallow dormancy for trees and shrubs, but to allow dormancy for forbs, you will provide a data frame to thedormargument instead of a single positive integer value. There will be two columns: 1) a “Species” column that has the species name for each species present indat, and 2) a column called “dorm” that has positive integer values indicating the dormancy you’d like to allow for each species. Make sure that if you are following the data frame approach, you must provide a dormancy argument for every species that has data indat. Make sure that the species names in thedormdata frame are spelled exactly the same as they are indat. The data frame should look something like this:
#> Species dorm
#> 1 tree A 0
#> 2 shrub B 0
#> 3 tree C 0
#> 4 forb D 1
#> 5 forb E 2
#> 6 forb F 1Important Note: Be very careful about how you define the
dormargument. The bigger thedormargument, the more likely you are to overestimate survival. For annually-sampled data, I would need a very biologically-compelling reason to to specify adormargument greater than 1 year.buffThis is a positive numeric value that indicates how much an individual can move from year 1 to year 2 and still be considered the same individual (receive the same trackID). In addition to accounting for true variation in location of a plant’s stem from year to year, this argument also accounts for small inconsistencies in mapping from year to year. Thebuffargument must be in the same units as the spatial values indat. For example, if the spatial data indatis measured in meters, and you want to allow a plant to “move” 15 cm between year 1 and year 2, then you would include the argumentbuff = .15in your call totrackSpp(). If you want to allow no movement, usebuff = 0. Below is a visualization of two differentbuffscenarios.
Figure 2.2: With a 10 cm buffer, these polygons in 1922 and 1923 overlap and will be identified by trackSpp() as the same individual and receive the same trackID.
Figure 2.3: With a 3 cm buffer, these polygons in 1922 and 1923 don’t quite overlap, so will be identified by trackSpp() as different individuals and receive different trackIDs.
-
clonalThis is a logical argument (TRUE or FALSE) that indicates whether you want to allow plants to be clonal or not. In the context of this type of data, “clonal” means that one genetic individual (or “genet”) can be recorded as multiple polygons (or “ramets”). Ifclonal = TRUE, then multiple polygons in the same year can be part of the same individual and have the same trackID. Ifclonal = FALSE, then every polygon in a given year is a unique individual and has a unique trackID. This option can be defined globally for all species present indatby settingclonalequal to FALSE or TRUE in thetrackSpp()function call. Alternatively,clonalcan be specified uniquely for each species by creating a data frame that contains aclonalargument for each species (analogous to the data frame for thedormargument shown in Table 2.1, but with a column called “clonal”).
The following arguments to trackSpp() are only required
in certain contexts.
-
buffGenetis an argument that is only required ifclonal = TRUEor ifclonalis a data frame that contains at least a singleTRUEin the “clonal” column.buffGenetis a numeric value that indicates how close polygons of the same species must be to one another in the first year in order to be considered parts of the same genetic individual (ramets of the same genet). Similar tobuff, be very careful and conservative when defining this argument. A large value forbuffGenetcan quickly lead to the entire quadrat being treated as the same genetic individual! I suggest experimenting with multiple values ofbuffGenet, looking at maps that show the trackID assignment, and deciding on a value that leads to trackID assignments that make the most biological sense to you. This argument is passed to thegroupByGenet()function, which assigns the same trackID to individuals that are within thebuffGenetbuffer of each other. Polygons are only grouped by genet in the first year of data. After that point, grouping by genet happens based on data in previous years. If there are multiple polygons that overlap with a genet in the previous year, they are given the same trackID and are considered ramets belonging to the same genet. The value ofbuffGenetmust be greater than or equal to zero, and must be in the same units as the spatial data indat.buffGenetcan be a single numeric value which will be applied to all species present indat, or can be specified uniquely for each species by creating a data frame that contains abuffGenetargument for each species (analogous to the data frame for thedormargument shown in Table 2.1, but with a column called “buffGenet”). -
aggByGenetis a logical argument that is only required ifclonal = TRUEor ifclonalis a data frame that contains at least a singleTRUEin the “clonal” column. This argument determines whether the output data frame fromtrackSpp()will have a row for every single ramet, or will be aggregated so that each genet is represented by a single row. IfaggByGenet = FALSE, then the output is not aggregated. IfaggByGenet = TRUE(the default setting), then the results are aggregated using theplantTrackerfunctionaggregateByGenet(). This function combines thesf“POLYGONS” for each ramet into onesfMULTIPOLYGONfor the entire genet, and combines the associated metadata (“Species”, “Site”, “Quad”, “Year”, “trackID”, “basalArea_genet”, “age”, “recruit”, “survives_tplus1”, “size_tplus1”, “nearEdge”) into one row for this genet. Even if the inputdathad additional columns, they will not be included in the output oftrackSppifaggByGenet = TRUE, since it is uncertain if they can be summed across all ramets or are identical across all ramets. For example, if each ramet has a unique character string in a column called “name”, there is no easy way to “sum” the character strings in this column to have one value for each genet. If you want the output data frame fromtrackSpp()to have the same columns as your inputdatdata.frame, set theaggByGenetargument to FALSE. However, Be Careful, since any demographic analysis should be done with a data.set that has only one row per genet, otherwise you will be estimating survival and growth rates on the scale of ramets instead of genets. If you take theaggByGenet = FALSEroute, be sure to pass your dataset through theaggregateByGenet()function (or aggregate to the genet scale using your preferred method) before demographic analysis. -
species/site/quad/year/geometryThese arguments only need to be included if the columns indatthat contain the data for species, site, quadrat, year and geometry of each observation are different from the names “Species”, “Site”, “Quad”, “Year, and”geometry”. For example, if the column in your version ofdatthat contains the species identity of each observation is called “species_names”, then the argumentspecies = "species_names"must be included in your call totrackSpp(). -
printMessagesThis is an optional logical argument that determines whether this function returns messages about genet aggregation, as well as messages indicating which year is the last year of sampling in each quadrat and which year(s) come before a gap in sampling that exceeds thedormargument (and thus which years of data have an “NA” for “survives_tplus1” and “size_tplus1”). IfprintMessages = TRUE(the default), then messages are printed. IfprintMessages = FALSE, then messages are not printed. -
flagSuspectsThis is an optional logical argument of length 1, indicating whether observations that are “suspect” will be flagged. The default isflagSuspects = FALSE. IfflagSuspects = TRUE, then a column called “Suspect” is added to the output data.frame. Any suspect observations get a “TRUE” in the “Suspect” column, while non-suspect observations receive a “FALSE”. There are two ways that an observation can be classified as “suspect”. First, if two consecutive observations have the same trackID, but the observation in year t+1 is less that a certain percentage (defined by theshrinkarg.) of the observation in year t, it is possible that the observation in year t+1 is a new recruit and not the same individual. The second way an observation can be classified as “suspect” is if it is very small before going dormant. It is unlikely that a very small individual will survive dormancy, so it is possible that the function has mistakenly given a survival value of “1” to this individual. A “very small individual” is any observation with an area below a certain percentile (specified bydormSize) of the size distribution for this species, which is generated using all of the size data for this species indat. If you are using the output dataset for demographic analysis, you may want to exclude “Suspect” observations. IfflagSuspects = FALSE, then no additional column is added. -
shrinkThis is an optional argument that takes a single numeric value. This value is only used whenflagSuspects = TRUE. When two consecutive observations have the same trackID, and the ratio of size_t+1 to size_t is smaller than the value ofshrink, the observation in year t gets a “TRUE” in the “Suspect” column. For example,shrink = 0.2, and an individual that the tracking function has identified as “BOUGRA_1992_5” has an area of 9 cm\(^2\) in year_t and an area of 1.35 cm\(^2\) in year_t+1. The ratio of size_t+1 to size_t is 1.35/9 = 0.15, which is smaller than the cutoff specified byshrink, so the observation of “BOUGRA_1992_5” in year t gets a “TRUE” in the “Suspect” column. The default value isshrink = 0.10. -
dormSizeThis is an optional argument that takes a single numeric value. This value is only used whenflagSuspects = TRUEanddorm ≥ 1. An individual is flagged as “suspect” if it “goes dormant” and has a size that is less than or equal to the percentile of the size distribution for this species that is designated bydormSize. For example,dormSize = 0.05, and an individual has a basal area of 0.5 cm\(^2\). The 5th percentile of the distribution of size for this species, which is made using the mean and standard deviation of all observations indatfor the species in question, is 0.6 cm\(^2\). This individual does not have any overlaps in the next year (year_t+1), but does have an overlap in year_t+2. However, because the basal area of this observation is smaller than the 5th percentile of size for this species, the observation in year t will get a “TRUE” in the “Suspect” column. It is possible that the tracking function has mistakenly assigned a “1” for survival in year_t, because it is unlikely that this individual is large enough to survive dormancy. The default value isdormSize = .05.
These are all of the possible arguments to
trackSpp()!
2.2 Function output
Below is an example of a potential function call to
trackSpp(), using the example dat and
inv data we’ve used so far. :
datTrackSpp <- plantTracker::trackSpp(dat = dat, inv = inv,
dorm = 1,
buff = .05,
buffGenet = .005,
clonal = data.frame("Species" = c("Heteropogon contortus",
"Bouteloua rothrockii",
"Ambrosia artemisiifolia",
"Calliandra eriophylla"),
"clonal" = c(TRUE,TRUE,FALSE,FALSE)),
aggByGenet = TRUE,
printMessages = FALSE
)And here’s what the output of this call to trackSpp()
looks like:
#> Simple feature collection with 477 features and 12 fields
#> Geometry type: GEOMETRY
#> Dimension: XY
#> Bounding box: xmin: -0.001386579 ymin: -0.001017592 xmax: 1.000536 ymax: 1.001267
#> CRS: NA
#> First 10 features:
#> Site Quad Species trackID Year type basalArea
#> 1 AZ SG2 Ambrosia artemisiifolia AMBART_1922_1 1922 point 2.461883e-05
#> 2 AZ SG2 Ambrosia artemisiifolia AMBART_1922_10 1922 point 2.461883e-05
#> 3 AZ SG2 Ambrosia artemisiifolia AMBART_1922_11 1922 point 2.461883e-05
#> 4 AZ SG2 Ambrosia artemisiifolia AMBART_1922_12 1922 point 2.461883e-05
#> 5 AZ SG2 Ambrosia artemisiifolia AMBART_1922_13 1922 point 2.461883e-05
#> 6 AZ SG2 Ambrosia artemisiifolia AMBART_1922_14 1922 point 2.461883e-05
#> 7 AZ SG2 Ambrosia artemisiifolia AMBART_1922_15 1922 point 2.461883e-05
#> 8 AZ SG2 Ambrosia artemisiifolia AMBART_1922_16 1922 point 2.461883e-05
#> 9 AZ SG2 Ambrosia artemisiifolia AMBART_1922_17 1922 point 2.461883e-05
#> 10 AZ SG2 Ambrosia artemisiifolia AMBART_1922_18 1922 point 2.461883e-05
#> recruit survives_t+1 age size_t+1 nearEdge geometry
#> 1 NA 0 NA NA TRUE POLYGON ((0.350604 0.021361...
#> 2 NA 0 NA NA FALSE POLYGON ((0.7172048 0.25834...
#> 3 NA 0 NA NA FALSE POLYGON ((0.1845598 0.38566...
#> 4 NA 0 NA NA FALSE POLYGON ((0.759387 0.399850...
#> 5 NA 0 NA NA FALSE POLYGON ((0.1696044 0.40291...
#> 6 NA 0 NA NA FALSE POLYGON ((0.7290925 0.41097...
#> 7 NA 0 NA NA FALSE POLYGON ((0.6255546 0.45737...
#> 8 NA 0 NA NA FALSE POLYGON ((0.5872073 0.46925...
#> 9 NA 0 NA NA FALSE POLYGON ((0.8863168 0.52256...
#> 10 NA 0 NA NA FALSE POLYGON ((0.5212498 0.52831...If you did not allow any species to be clonal
(clonal = 0) or if aggByGenet = TRUE in your
call to trackSpp(), then your output data frame will have
one row for each genet, and is ready for demographic analysis! If your
output data frame is not yet aggregated by genet (i.e. you use
aggByGenet = FALSE), then you need to transform your data
frame so that each genet is represented by only one row of data. You can
use the aggregateByGenet() function from
plantTracker (see this function’s documentation for
guidance), or your own method of choice.
You can stop here and proceed to your own analyses using the
demographic data you generated, or you can proceed with other
plantTracker functions outlined below for some additional
useful data.
3 Calculate local neighborhood density using
getNeighbors()
It is often useful in demographic analyses to have some idea of the competition (or facilitation) that an individual organism is dealing with. Interactions between individuals can have a profound impact on whether an organism survives and grows. Spatial datasets of plant occurrence allow us to generate an estimate of the interactions an individual plant has with other plants by determining how many other individuals occupy the “local neighborhood” of each focal plant. While this isn’t a direct measure of competition or facilitation, it gives us an estimate that we can include in demographic models.
Here is the generic getNeighbors() function:
getNeighbors(dat, buff, method, compType = "allSpp", output = "summed",
trackID = "trackID", species = "Species", quad = "Quad", year = "Year",
site = "Site", geometry = "geometry", ...)3.1 Function options and arguments
The getNeighbors() function in plantTracker
calculates local neighborhood density for each unique individual in your
dataset. A user-specified buffer is drawn around each individual, and
then the function counts the number of other plants within this
buffer.This function can only be run on a dataset where each unique
individual (genet) is represented by only one row of data. If the genet
consists of multiple polygons, then they must be aggregated into one
sf MULTIPOLYGON object. If your dataset has
multiple rows for each genet, then you can use the
aggregateByGenet() function to get it ready to use in
getNeighbors(). Additionally, getNeighbors()
requires your dataset to have a column containing a unique identifier
for each genet. Across multiple years, that genet must have the same
unique identifier. If you are using this function right after
trackSpp(), your dataset will already have this unique
identifier in a column called “trackID”.
getNeighbors() has several options that allow you to
customize how local neighborhood density is calculated.
- First, the user can decide how the function “counts” other plants
inside the buffer zone around the focal individual. Option 1) The
function will
tally the number of genets inside the buffer zone. Option 2) The function will calculate the proportion of the buffer zone that is occupied by other plants. - Second, the user can decide whether the function will
calculate
intraspecific local neighborhood density (only consider other plants in the buffer zone of the same species as the focal individual) or interspecific local neighborhood density (consider all other plants in the buffer zone, regardless of species). - Third, the user can decide whether the neighborhood density value (either a count or area) for each focal individual is a single value that sums the number or area of neighbors, or whether it is actually a list that provides the neighborhood density for each species present in the neighborhood.
Figure 3.1: This individual outlined in pink is a focal individual, and the pale pink shows a 10 cm buffer around it.
Figure 3.2: The 10cm buffer around the focal
individual overlaps with 5 other unique individuals of two species.
These overlapping individuals are outlined in dark grey. Using the
‘count’ method in getNeighbors(), we would get an
intraspecific competition value of 3, and an interspecific competition
value of 5.
Figure 3.3: The 10cm buffer around the focal
individual overlaps with 5 other unique individuals of two species. The
overlapping area is shaded in grey. Using the ‘area’ method in
getNeighbors(), we would get an intraspecific competition
metric of 0.0454, and an interspecific competition metric of
0.0462.
Below are the arguments in the getNeighbors()
function.
-
datAnsfdata frame in which each row represents data for a unique individual organism in a unique year. Thesfgeometry for each row must be eitherMULTIPOLYGONorPOLYGONgeometry. In addition to a “geometry” column, this data frame must have columns that contain data indicating the site, quadrat, site, and year of each observation. There also must be a column that contains a unique identifying value for each genet in each year. Ifdatis coming directly fromtrackSpp(), this column will be called “trackID”.
-
buffThis is a single numeric value that indicates the desired width of the “buffer” around the focal individual in which the competitors are to be counted. This value must be in the same units as the spatial information indat.
-
methodThis is a character string that must equal either"count"or"area". Ifmethod = "count", then the number of individuals in the buffer area will be tallied. Ifmethod = "area", then the proportion of the buffer area that is occupied by other individuals will be calculated. -
compTypeThis is a character string that must be either"allSpp"or"oneSpp". IfcompType = "allSpp", then a metric of interspecific competition is calculated, meaning that every individual within the buffer around the focal individual is considered, no matter the species. IfcompType = "oneSpp", then a metric of intraspecific competition is calculated, meaning that only individuals of the same species as the focal individual will be considered when calculating the competition metric. If no value is provided, it will default to “allSpp”. -
outputThis is a character string that is set to either"summed"or"bySpecies". The default is"summed". This argument is only important to consider if you are usingcompType = "allSpp". Ifoutput = "summed", then only one count/area value is returned for each individual. This value is the total count or area of all neighbors within the focal species buffer zone, regardless of species. Ifoutput = "bySpecies", there is a count or area value returned for each species present in the buffer zone. For example, you are usinggetNeighbors()withmethod = "count"andcompType = "allSpp". A focal individual in your dataset has seven other plants inside its buffer zone, three of species A, two of species B, and 2 of species C. Ifoutput = "summed", the value in the “neighbors_count” column of the returned data frame will simply contain the value “7”. Ifoutput = "bySpecies", the “neighbors_count” column for this individual will actually contain a named list{r} list("Species A "= 5, "Species B" = 3, "Species C" = 7). The default value ofoutputis"summed".
-
trackID/species/quad/year/site/geometryThese arguments only need to be included if the columns indatthat contain the data for trackID, species, site, quadrat, year and geometry of each observation are different from the names “trackID,”Species”, “Site”, “Quad”, “Year, and”geometry”. For example, if the column in your version ofdatthat contains the species identity of each observation is called “species_names”, then the argumentspecies = "species_names"must be included in your call togetNeighbors().
3.2 Function outputs
The output of getNeighbors() is an sf data
frame that is identical to the input dat, but with either
one or two additional columns. If method = "area", there
are two columns added called “nBuff_area” and “neighbors_area”. The
first contains the area of the buffer zone around each focal individual.
The second contains the basal area of neighbors that overlap with a
focal individual’s buffer zone. If method = "count", there
is only one additional column added to the output, called
“neighbors_count.” This column contains a count of the neighbors that
occur within a focal individual’s buffer zone.
Here’s an example of a getNeighbors() function call
using the resulting data from the example in section 2.2, as
well as the resulting data frame. Note that
method = "area", so two columns are added to the returned
data frame:
datNeighbors <- plantTracker::getNeighbors(dat = datTrackSpp,
buff = .15,
method = "area",
compType = "allSpp")#> Simple feature collection with 477 features and 14 fields
#> Geometry type: GEOMETRY
#> Dimension: XY
#> Bounding box: xmin: -0.001386579 ymin: -0.001017592 xmax: 1.000536 ymax: 1.001267
#> CRS: NA
#> First 10 features:
#> Species Site Quad trackID Year neighbors_area
#> 1 Ambrosia artemisiifolia AZ SG2 AMBART_1922_1 1922 0.0011328591
#> 2 Ambrosia artemisiifolia AZ SG2 AMBART_1922_10 1922 0.0040518140
#> 3 Ambrosia artemisiifolia AZ SG2 AMBART_1922_11 1922 0.0055774468
#> 4 Ambrosia artemisiifolia AZ SG2 AMBART_1922_12 1922 0.0029129080
#> 5 Ambrosia artemisiifolia AZ SG2 AMBART_1922_13 1922 0.0050297901
#> 6 Ambrosia artemisiifolia AZ SG2 AMBART_1922_14 1922 0.0037646754
#> 7 Ambrosia artemisiifolia AZ SG2 AMBART_1922_15 1922 0.0026270846
#> 8 Ambrosia artemisiifolia AZ SG2 AMBART_1922_16 1922 0.0013297048
#> 9 Ambrosia artemisiifolia AZ SG2 AMBART_1922_17 1922 0.0022690175
#> 10 Ambrosia artemisiifolia AZ SG2 AMBART_1922_18 1922 0.0006338116
#> nBuff_area basalArea basalArea_genet survives_t+1 survives_tplus1 size_t+1
#> 1 0.04344697 point 2.461883e-05 NA 0 NA
#> 2 0.07329218 point 2.461883e-05 NA 0 NA
#> 3 0.07329218 point 2.461883e-05 NA 0 NA
#> 4 0.07329218 point 2.461883e-05 NA 0 NA
#> 5 0.07329218 point 2.461883e-05 NA 0 NA
#> 6 0.07329218 point 2.461883e-05 NA 0 NA
#> 7 0.07329218 point 2.461883e-05 NA 0 NA
#> 8 0.07329218 point 2.461883e-05 NA 0 NA
#> 9 0.06848976 point 2.461883e-05 NA 0 NA
#> 10 0.07329218 point 2.461883e-05 NA 0 NA
#> size_tplus1 nearEdge geometry
#> 1 NA TRUE POLYGON ((0.350604 0.021361...
#> 2 NA FALSE POLYGON ((0.7172048 0.25834...
#> 3 NA FALSE POLYGON ((0.1845598 0.38566...
#> 4 NA FALSE POLYGON ((0.759387 0.399850...
#> 5 NA FALSE POLYGON ((0.1696044 0.40291...
#> 6 NA FALSE POLYGON ((0.7290925 0.41097...
#> 7 NA FALSE POLYGON ((0.6255546 0.45737...
#> 8 NA FALSE POLYGON ((0.5872073 0.46925...
#> 9 NA FALSE POLYGON ((0.8863168 0.52256...
#> 10 NA FALSE POLYGON ((0.5212498 0.52831...The example above uses the option output = "summed",
which is the default for the getNeighbors() function. With
this option, the “neighbors_area” or “neighbors_count” column (depending
on the method argument) contains just one value that sums
the neighbor count or area across all neighbor species. However, if
output = "bySpecies", the “neighbors_count” or
“neighbors_area” column contains a list with the counts or areas broken
down by species. The output argument is described in more
detail in section 3.1. If you want to use the
getNeighbors() function to determine how the effect of
neighbors differs according to the species identity of those neighbors,
setting output = "bySpecies" allows you to do this.
However, it is likely that your subsequent analyses will need the
by-species neighbor data in a matrix or data frame format, rather than
in a list nested inside a data frame, which is how
getNeighbors() returns the data.
Here is some code that turns the data returned by
getNeighbors(output = "bySpecies") into a matrix where each
row has data for one focal individual, and each column has data for one
species. This format should be easier to work with for further
analysis.
# save the output of the getNeighbors() function
datNeighbors_bySpp <- plantTracker::getNeighbors(dat = datTrackSpp,
buff = .15, method = "area", compType = "allSpp", output = "bySpecies")
# determine all of the possible species that can occupy the buffer zone
compSpp <- unique(datTrackSpp$Species)
temp <- lapply(X = datNeighbors_bySpp$neighbors_area, FUN = function(x) {
tmp <- unlist(x)
tmp2 <- tmp[compSpp]
}
)
for (i in 1:length(temp)) {
# fix the column names
names(temp[[i]]) <- compSpp
# save the data in a matrix
if (i == 1) {
datOut <- temp[[i]]
} else {
datOut <- rbind(datOut, temp[[i]])
}
}
# make the rownames of the matrix correspond to the trackID of the focal individual
rownames(datOut) <- datNeighbors_bySpp$trackID
# show the first few rows of the datOut data frame:
datOut[1:5,]
#> Ambrosia artemisiifolia Bouteloua rothrockii
#> AMBART_1922_1 4.923767e-05 5.216672e-04
#> AMBART_1922_10 8.437672e-05 1.250163e-03
#> AMBART_1922_11 2.461883e-05 2.310445e-04
#> AMBART_1922_12 9.353468e-05 7.408342e-04
#> AMBART_1922_13 2.461883e-05 1.906272e-08
#> Calliandra eriophylla Heteropogon contortus
#> AMBART_1922_1 NA 0.0005619542
#> AMBART_1922_10 NA 0.0027172740
#> AMBART_1922_11 NA 0.0053217835
#> AMBART_1922_12 NA 0.0020785392
#> AMBART_1922_13 NA 0.00500515224 Next steps
At this point, this dataset should be ready for you to use in any applications wish! There are a few additional functions that may help you in your analyses, and these are outlined in this section.
- Calculate recruitment by species-by-plot-by-year: the
getRecruits()function - Calculate population rate of increase (lambda) for each species in a
plot: the
getLambda()function - Calculate the basal area of each species in each quadrat in each
year: the
getBasalAreas()function - Make maps of quadrats over time: the
drawQuadMap()function
Specific instructions for how to use each of these functions can be found in their documentation!